Extra 15% OFF Health & Wellness
Oncology
Buy Lynparza (Olaparib) Online
0 out of 5
Lynparza is an oral medication used to treat some types of cancer by blocking the action of the PARP enzyme, preventing cancer cells from multiplying. It is used for ovarian, breast, and pancreatic cancer patients with a BRCA gene mutation or triple-negative breast cancer. Lynparza has possible side effects such as fatigue, nausea, diarrhea, and loss of appetite; it can also increase the risk of other cancers such as leukemia. Pregnant or breastfeeding women should not use Lynparza. The cost can be high, but there are programs to help patients. Lynparza is an important targeted therapy treatment option for cancers with a genetic component.
$5,294.30
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
Buy Ninlaro (ixazomib) Online
0 out of 5
DISEASE INDICATIONS: Multiple Myeloma MANUFACTURER: Takeda Pharma A/S USAGE: Oral MEDICINE APPROVED BY: European Medical Agency (EMA) Food and Drug Administration (FDA) Health Canada Pharmaceuticals and Medical Devices Agency (PMDA) Therapeutic Goods Administration (TGA) Ninlaro (ixazomib) is a medication used in combination with lenalidomide and dexamethasone to treat people with multiple myeloma who have undergone one to three previous lines of therapy.
$5,985.10
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
Buy Odomzo (sonidegib) Online
0 out of 5
DISEASE INDICATIONS: Skin Cancer MANUFACTURER: Sun Pharmaceutical Industries Europe B.V. USAGE: Oral MEDICINE APPROVED BY: European Medical Agency (EMA) Food and Drug Administration (FDA) Therapeutic Goods Administration (TGA) Odomzo (sonidegib) is a medication used to treat locally advanced basal cell carcinoma (BCC). It is taken once daily at two possible dosages - 200mg or 800mg.
$5,478.00
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
Buy Onivyde (irinotecan hydrochloride trihydrate) Online
0 out of 5
DISEASE INDICATIONS: Pancreatic Cancer MANUFACTURER: Baxalta Innovations GmbH USAGE: Intravenous MEDICINE APPROVED BY: European Medical Agency (EMA) Food and Drug Administration (FDA) Health Canada Therapeutic Goods Administration (TGA) Medsafe (NZ) Onivyde (irinotecan hydrochloride trihydrate) is a chemotherapy drug used to treat metastatic adenocarcinoma of the pancreas that has worsened despite previous cancer treatment containing gemcitabine.
$1,037.30
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
Buy Perjeta (Pertuzumab) Online
0 out of 5
Perjeta is a monoclonal antibody used in the treatment of HER2-positive breast cancer. It works by preventing the HER2 receptor from interacting with other receptors. It belongs to the class of drugs known as HER2 inhibitors and is FDA approved to be used in combination with Herceptin and chemotherapy. Perjeta has been shown to significantly improve progression-free survival in patients with metastatic HER2-positive breast cancer. It is administered intravenously once every three weeks and can be continued up to a year or more in certain patients who are responding well to treatment. Common side effects of Perjeta include fatigue, nausea, diarrhea, and hair loss.
$2,557.50
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
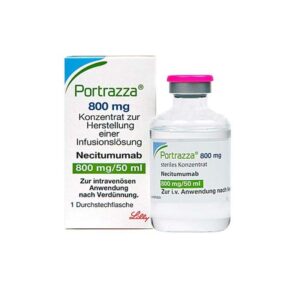
Buy Portrazza (necitumumab) Online
0 out of 5
DISEASE INDICATIONS: Lung Cancer MANUFACTURER: Eli Lilly Nederland B.V. USAGE: Intravenous MEDICINE APPROVED BY: European Medical Agency (EMA) Food and Drug Administration (FDA) Health Canada Portrazza (necitumumab) is a medication approved for the treatment of advanced squamous non-small cell lung cancer. It is used in combination with the chemotherapies gemcitabine and cisplatin.
$7,736.30
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
Buy Rubraca (rucaparib) Online
0 out of 5
From $1,691.80
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
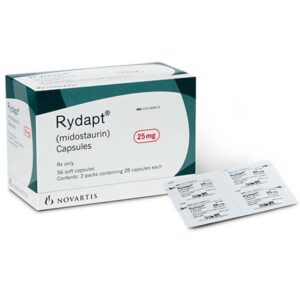
Buy Rydapt (midostaurin) Online
0 out of 5
DISEASE INDICATIONS: Leukemia MANUFACTURER: Novartis Europharm Limited USAGE: Oral MEDICINE APPROVED BY: European Medical Agency (EMA) Food and Drug Administration (FDA) Health Canada Therapeutic Goods Administration (TGA) Medsafe (NZ) Rydapt (midostaurin) is prescribed to treat newly diagnosed acute myeloid leukemia (AML), aggressive systemic mastocytosis (ASM), systemic mastocytosis with associated hematological neoplasm (SM-AHN), or mast cell leukemia (MCL) in patients.
$14,685.00
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
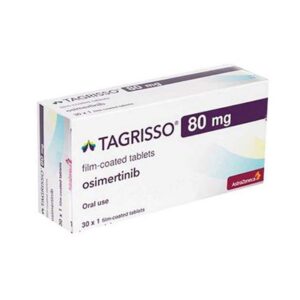
Buy Tagrisso (Osimertinib) Online
0 out of 5
- DISEASE INDICATIONS: Lung Cancer
- MANUFACTURER: AstraZeneca AB
- USAGE: Oral
- MEDICINE APPROVED BY:
- European Medical Agency (EMA)
- Food and Drug Administration (FDA)
- Health Canada
- Therapeutic Goods Administration (TGA)
- Medsafe (NZ)
$5,720.00
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
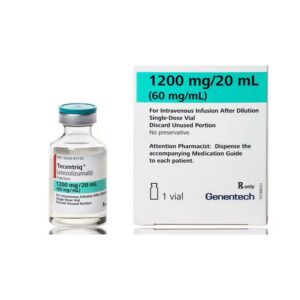
Buy Tecentriq (Atezolizumab) Online
0 out of 5
Tecentriq (Atezolizumab) is an immune checkpoint inhibitor used in conjunction with chemotherapy or other cancer medications to attack cancer cells. By blocking PD-L1, a protein on some cancer cells, Tecentriq allows the immune system to recognize and target cancer cells. Clinical trials show that Tecentriq extends survival and improves outcomes in patients with lung cancer, bladder cancer, and triple-negative breast cancer. Tecentriq is generally well-tolerated, administered intravenously once every three or four weeks, and monitored for potential side effects. Tecentriq is FDA-approved for several types of cancer, making it a promising treatment option for many cancer patients.
$6,268.90
Select optionsThis product has multiple variants. The options may be chosen on the product page
Oncology
Buy Zejula (niraparib) Online
0 out of 5
DISEASE INDICATIONS: Gynaecological Cancer MANUFACTURER: Tesaro UK Limited USAGE: Oral MEDICINE APPROVED BY: European Medical Agency (EMA) Food and Drug Administration (FDA) Health Canada Therapeutic Goods Administration (TGA) Zejula (niraparib) is a drug used for the maintenance treatment of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in adult patients who have responded to platinum-based chemotherapy.
From $5,017.10
Select optionsThis product has multiple variants. The options may be chosen on the product page