- Your cart is empty
- Continue Shopping

Product
Buy FEMARA 2.5MG (LETROZOLE) – NOVARTIS Online
$46.76Current price is: $46.76. Original price was: $52.53.
CHARACTERISTICS
ACTIVE HALF-LIFE 2 DAYS
CLASSIFICATION NON-STEROIDAL AROMATASE INHIBITOR
DOSAGE 0.5 – 2.5 MG/DAY
ACNE NO
WATER RETENTION NO
HBR NO
HEPATOTOXICITY NO
AROMATIZATION NO
ACTIVE SUBSTANCE LETROZOLE
FORM 30 PILLS X 2.5 MG
MANUFACTURER NOVARTIS
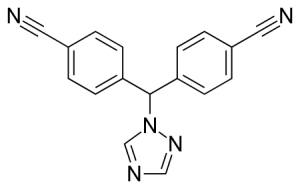
Chemical name: 4-[(4-cyanophenyl)-(1,2,4-triazol-1-yl)methyl]benzonitrile;
Formula: C17H11N5
Introduction
FEMARA is a medication manufactured by Novartis, containing letrozole as its active substance. It belongs to the class of medications known as aromatase inhibitors and is primarily used in the treatment of hormone receptor-positive breast cancer in postmenopausal women. This description aims to provide an overview of FEMARA, its characteristics, clinical applications, and considerations.
Characteristics
- Active Substance: FEMARA contains letrozole, a potent aromatase inhibitor that works by blocking the enzyme aromatase, which is responsible for converting androgens into estrogen. By inhibiting estrogen production, letrozole helps reduce estrogen levels in the body, thereby slowing down the growth of hormone-sensitive breast cancer cells.
- Formulation: FEMARA is available in the form of tablets, with each tablet containing a specific dosage of letrozole. The tablets are typically film-coated and may be scored for ease of administration. Common dosages include 2.5 milligrams per tablet.
Clinical Applications
- Breast Cancer Treatment: FEMARA is indicated for the adjuvant treatment of hormone receptor-positive early breast cancer in postmenopausal women who have received prior standard adjuvant tamoxifen therapy. It is also used for the extended adjuvant treatment of early breast cancer after completion of five years of adjuvant tamoxifen therapy.
- Advanced Breast Cancer: In addition to its use in the adjuvant setting, FEMARA is also indicated for the treatment of advanced breast cancer (metastatic breast cancer) in postmenopausal women who have progressed following antiestrogen therapy, such as tamoxifen.
Considerations
- Dosage and Administration: The recommended dosage of FEMARA may vary depending on the specific indication and individual patient factors. It is typically administered orally once daily, with or without food. Treatment duration may vary, and patients should follow their healthcare provider’s instructions regarding dosing and duration of therapy.
- Monitoring: During treatment with FEMARA, patients may undergo regular monitoring to assess treatment response, disease progression, and potential adverse effects. This may include clinical examinations, imaging studies, laboratory tests, and assessments of bone mineral density.
- Adverse Effects: Common adverse effects associated with FEMARA may include hot flashes, arthralgia (joint pain), fatigue, bone pain, headache, and nausea. Rare but more serious side effects may include osteoporosis, fractures, cardiovascular events, and liver toxicity. Patients should report any new or worsening symptoms to their healthcare provider promptly.
- Bone Health: Long-term use of aromatase inhibitors like FEMARA may increase the risk of osteoporosis and fractures due to estrogen depletion. Healthcare providers may recommend measures to promote bone health, such as calcium and vitamin D supplementation, weight-bearing exercises, and bone density monitoring.
- Hormone Receptor Status: FEMARA is specifically indicated for the treatment of hormone receptor-positive breast cancer. Before initiating treatment, patients may undergo testing to determine the hormone receptor status of their tumor to ensure appropriate selection of therapy.
Conclusion
FEMARA, containing letrozole as its active substance, is a valuable medication in the treatment of hormone receptor-positive breast cancer in postmenopausal women. By inhibiting estrogen production, FEMARA helps slow down the growth of breast cancer cells and improve treatment outcomes. However, its use requires careful consideration of dosage, duration of therapy, and potential risks, and should be guided by a qualified healthcare provider to ensure safe and effective treatment outcomes.
| manufacturer |
|---|







Reviews
There are no reviews yet.