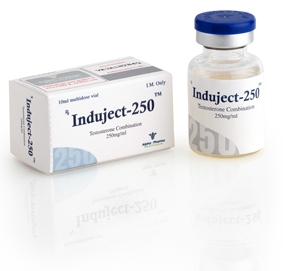

Buy INDUJECT-250 Online
$39.71
CHARACTERISTICS
ACTIVE HALF-LIFE 7-8 DAYS
CLASSIFICATION ANABOLIC STEROID
DOSAGE MEN 300-1500 MG/WEEK
ACNE YES
WATER RETENTION YES
HBR PERHAPS
HEPATOTOXICITY NO
AROMATIZATION YES
ACTIVE SUBSTANCE TESTOSTERONE DECANOATE, TESTOSTERONE ISOCAPROATE, TESTOSTERONE PHENYLPROPIONATE, TESTOSTERONE PROPIONATE
FORM 10 ML X 250 MG/ML
MANUFACTURER ALPHA PHARMA
Buy INDUJECT-250 Online
$39.71
- Description
- Reviews (0)
Description
Chemical name: (17β)-3-Oxoandrost-4-en-17-yl propanoate
Formula: C22H32O3
Anabolic activity index: 100% (reference drug)
Androgenic activity index: 100% (reference drug)
INDUJECT-250 10ml VIAL – ALPHA PHARMA
Description: INDUJECT-250 is a medication manufactured by Alpha Pharma. It is available in a 10ml vial and is formulated as an injectable solution. INDUJECT-250 is a combination product containing several testosterone esters, which are synthetic derivatives of the male sex hormone testosterone.
Uses: INDUJECT-250 is primarily prescribed for the treatment of hypogonadism (low testosterone levels) in men. It may also be used in hormone replacement therapy (HRT) for transgender men, and in certain cases of delayed puberty in adolescent males.
How to Use: INDUJECT-250 is administered via intramuscular injection. The dosage and frequency of administration depend on the individual patient’s medical condition, testosterone levels, and response to treatment. Administration should be performed by a healthcare professional according to proper injection techniques.
Storage Conditions: INDUJECT-250 vials should be stored at room temperature, away from moisture, heat, and direct sunlight. It is important to keep the medication out of reach of children and pets.
Mechanism of Action: INDUJECT-250 contains a blend of testosterone esters, including testosterone propionate, testosterone phenylpropionate, testosterone isocaproate, and testosterone decanoate. These esters are absorbed at different rates into the bloodstream after injection, providing both immediate and sustained release of testosterone. Testosterone replacement therapy helps restore testosterone levels in individuals with low levels of this hormone.
Precautions:
- Patients with a history of prostate cancer, breast cancer, or cardiovascular disease should use INDUJECT-250 cautiously and under the supervision of a healthcare provider.
- Regular monitoring of testosterone levels and clinical response is important during treatment.
Contraindications: INDUJECT-250 is contraindicated in individuals with hypersensitivity to testosterone or any component of the formulation. It should not be used in women, particularly pregnant or breastfeeding women, due to the risk of masculinization and potential harm to the fetus or nursing infant.
Drug Interactions: INDUJECT-250 may interact with certain medications, particularly anticoagulants, insulin, and oral hypoglycemic agents. Patients should inform their healthcare provider about all medications they are taking to avoid potential interactions.
Side Effects: Common side effects of INDUJECT-250 may include acne, oily skin, hair loss, increased body hair growth, and mood changes. Other potential side effects include fluid retention, gynecomastia (enlarged breast tissue in men), and changes in libido. Serious adverse reactions such as liver toxicity, cardiovascular events, and prostate enlargement may occur but are rare.
Patients should consult their healthcare provider for personalized advice and information regarding INDUJECT-250, including its uses, dosage, and potential side effects.
Be the first to review “Buy INDUJECT-250 Online” Cancel reply
Related Products
Buy SUSTANON 350 10ml VIAL - DRAGON PHARMA Online
Total Sales: 0
SKU: 139564
Buy TESTO P (TESTOSTERONE PROPIONATE) Online
Total Sales: 0
SKU: 814228
Buy TESTOBOLIN (TESTOSTERONE ENANTHATE) Online
Total Sales: 0
SKU: 395819
Buy TESTOBOLIN AMP (TESTOSTERONE ENANTHATE) Online
Total Sales: 0
SKU: 224286
Buy TESTOSTERONE ENANTHATE 250 MG Online
Total Sales: 0
SKU: 557448

Reviews
There are no reviews yet.