- Your cart is empty
- Continue Shopping

Product
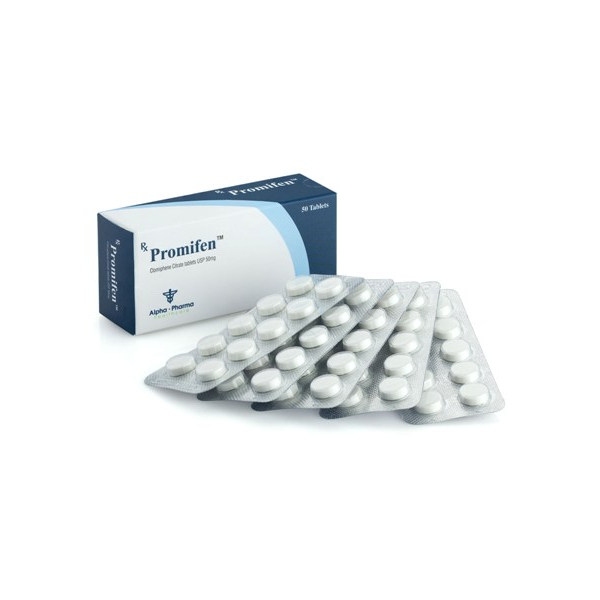
Buy PROMIFEN 50mg (CLOMIPHENE CITRATE) – ALPHA PHARMA Online
$23.69
CHARACTERISTICS
ACTIVE HALF-LIFE 5-7 DAYS
CLASSIFICATION SERM
DOSAGE 50-100 MG/DAY
ACNE NO
WATER RETENTION NO
HBR RARE
HEPATOTOXICITY LOW
AROMATIZATION NO
ACTIVE SUBSTANCE CLOMIPHENE CITRATE
FORM 50 PILLS X 50 MG
MANUFACTURER ALPHA PHARMA
Chemical name: 2-[4-(2-chloro-1,2-diphenylethenyl)phenoxy]-~{N},~{N}-diethylethanamine
Formula: C26H28ClNO
Anabolic activity index: not a steroid
Androgenic activity index: not a steroid
PROMIFEN 50mg – ALPHA PHARMA
1. Active Substance:
- Clomiphene Citrate: Clomiphene Citrate is the active pharmaceutical ingredient in Promifen 50mg tablets. It is a selective estrogen receptor modulator (SERM) that is used primarily in the treatment of infertility in women and to stimulate ovulation. Clomiphene Citrate works by blocking estrogen receptors in the hypothalamus, leading to an increase in follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which in turn stimulates ovulation.
2. Indications:
- Female Infertility: Promifen is indicated for the treatment of ovulatory dysfunction in women who wish to become pregnant. It is commonly used in women with polycystic ovary syndrome (PCOS) or unexplained infertility.
- Off-label Use in Men: Clomiphene Citrate is sometimes used off-label in men with hypogonadism to stimulate the production of testosterone and improve fertility.
3. Dosage:
- Each Promifen 50mg tablet contains 50 mg of Clomiphene Citrate.
- The recommended dosage and duration of treatment may vary depending on the individual patient’s response to therapy, medical history, and underlying cause of infertility.
- Treatment is usually initiated with a low dose, which may be gradually increased in subsequent cycles if ovulation does not occur.
4. Administration:
- Promifen tablets are taken orally with a full glass of water.
- The tablets can be taken with or without food.
- It is important to follow the dosage instructions provided by a healthcare professional.
5. Effectiveness:
- Promifen is effective in inducing ovulation in women with ovulatory dysfunction, leading to increased chances of conception.
- In men with hypogonadism, Clomiphene Citrate may help restore normal testosterone levels and improve fertility.
6. Safety Profile:
- Promifen is generally well-tolerated when used as prescribed. Common side effects may include hot flashes, mood swings, breast tenderness, nausea, and headache.
- Serious side effects such as ovarian hyperstimulation syndrome (OHSS), visual disturbances, and multiple pregnancies are rare but possible.
- Patients should be monitored closely for signs of ovarian enlargement or hyperstimulation during treatment.
7. Contraindications:
- Promifen is contraindicated in patients with known hypersensitivity to Clomiphene Citrate or any of the excipients in the formulation.
- It should not be used in patients with liver disease, ovarian cysts, or abnormal uterine bleeding of unknown origin.
8. Precautions:
- Promifen should be used with caution in patients with a history of thromboembolic disorders or endometriosis.
- It should be avoided in pregnant or breastfeeding women due to the risk of harm to the fetus or infant.
9. Storage:
- Promifen should be stored at room temperature (15°C to 30°C) in a dry place, away from moisture and heat.
- Keep the medication out of reach of children and pets.
Promifen 50mg tablets, manufactured by Alpha Pharma, offer an effective treatment option for women with ovulatory dysfunction and men with hypogonadism. Treatment should be initiated and monitored by a qualified healthcare professional, and patients should be informed about potential side effects and precautions associated with Clomiphene Citrate therapy.








Reviews
There are no reviews yet.