- Your cart is empty
- Continue Shopping

Product
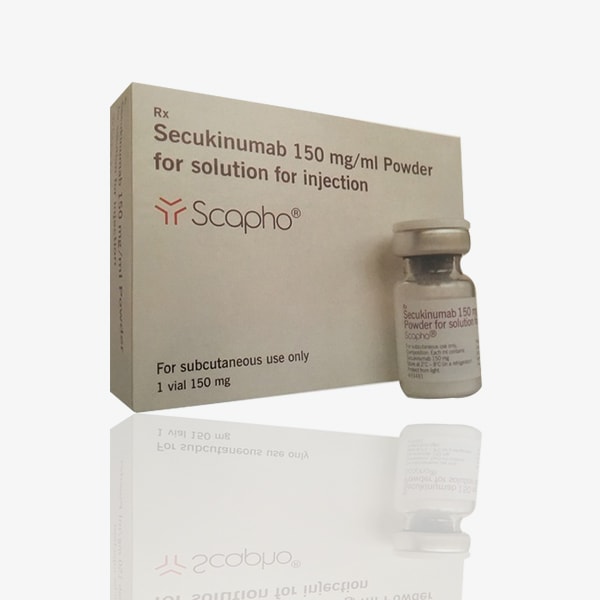
Scapho is a medication produced by Novartis India Ltd., and imported by Sandoz Private Limited India. It is available in the form of an injection containing the active ingredient secukinumab at a strength of 150 mg/ml. Here is a detailed description of Scapho:
Composition:
- Scapho contains the active ingredient secukinumab. Secukinumab is a monoclonal antibody that targets and inhibits interleukin-17A (IL-17A), a pro-inflammatory cytokine involved in the pathogenesis of autoimmune diseases such as psoriasis, psoriatic arthritis, and ankylosing spondylitis.
Indications:
- Scapho (secukinumab) is indicated for the treatment of various autoimmune conditions, including:
- Plaque Psoriasis: It is used to treat moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
- Psoriatic Arthritis: Scapho is indicated for the treatment of active psoriatic arthritis, either alone or in combination with other disease-modifying antirheumatic drugs (DMARDs).
- Ankylosing Spondylitis: It is also approved for the treatment of active ankylosing spondylitis in adults who have responded inadequately to conventional therapy.
Dosage and Administration:
- The recommended dosage and administration schedule for Scapho may vary depending on the specific indication being treated and the patient’s individual response to therapy. It is administered as a subcutaneous injection, typically once weekly for the first month, followed by maintenance dosing every four weeks thereafter.
Efficacy:
- Clinical studies have demonstrated the efficacy of secukinumab in improving the signs and symptoms of plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis, including reductions in skin lesions, joint pain, and inflammation. It provides rapid and sustained relief for patients with these chronic autoimmune conditions.
Side Effects:
- Common side effects of Scapho may include injection site reactions (such as pain, redness, or swelling), upper respiratory tract infections, headache, and diarrhea. Serious adverse reactions, including infections and hypersensitivity reactions, may occur in some individuals.
Precautions:
- Before starting Scapho therapy, patients should undergo a thorough medical evaluation, including assessment of baseline laboratory parameters and screening for latent tuberculosis. It should be used with caution in patients with a history of recurrent infections or immunodeficiency.
Contraindications:
- Scapho is contraindicated in individuals with a known hypersensitivity to secukinumab or any of the other components of the medication. It should not be used in patients with active infections, including tuberculosis.
Storage:
- Scapho injections should be stored as per the manufacturer’s recommendations, protecting them from light and excessive heat, and stored at the recommended temperature.
Scapho (secukinumab) offers a targeted and effective treatment option for individuals with moderate to severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis, providing relief from symptoms and improving quality of life. However, its use should be carefully managed and monitored by qualified healthcare professionals to ensure optimal safety and efficacy for individual patients. Regular follow-up visits and monitoring may be necessary during treatment.







Reviews
There are no reviews yet.