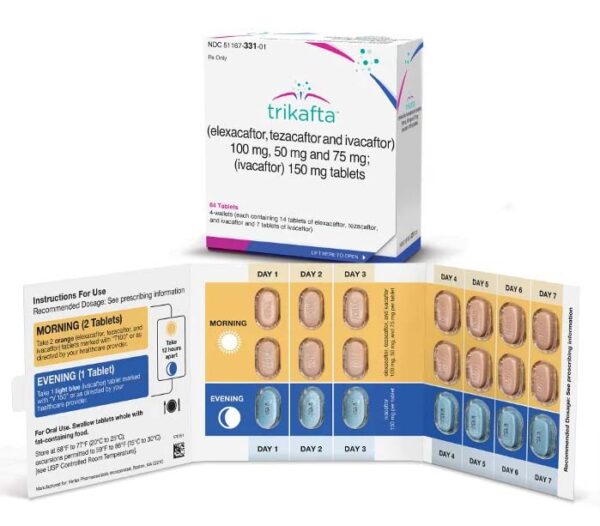

Buy Trikafta (elexacaftor/tezacaftor/ivacaftor; ivacaftor) Online
From $40,150.00
- DISEASE INDICATIONS: Cystic Fibrosis
- MANUFACTURER: Vertex Pharmaceuticals Incorporated
- USAGE: Oral
- MEDICINE APPROVED BY:
- Food and Drug Administration (FDA)
Trikafta (elexacaftor/tezacaftor/ivacaftor) is a medication used to treat cystic fibrosis in patients aged 12 years and older who have at least one F508del mutation in the CFTR gene.
Buy Trikafta (elexacaftor/tezacaftor/ivacaftor; ivacaftor) Online
From $40,150.00
- Description
- Additional information
- Reviews (0)
Description
Introduction
Trikafta (elexacaftor/tezacaftor/ivacaftor) is a medication used to treat cystic fibrosis, a genetic disease that affects the lungs, pancreas, and other organs. Trikafta is a combination of three drugs: elexacaftor, tezacaftor, and ivacaftor. The drug is approved by the US Food and Drug Administration (FDA) for the treatment of cystic fibrosis in patients aged 12 years and older who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.
Uses
Trikafta is a combination of drugs that work together to address the underlying genetic defect in cystic fibrosis. Elexacaftor helps to stabilize the defective protein produced by the CFTR gene, while tezacaftor increases the amount of functional protein present on the cell surface. Ivacaftor helps to enhance the activity of the CFTR protein once it reaches the cell surface. By addressing these different aspects of CFTR function, Trikafta can improve lung function and reduce the occurrence of respiratory infections in patients with cystic fibrosis.
How it Works
The CFTR protein is responsible for regulating the flow of salt and water in and out of cells in the lungs, pancreas, and other organs. In cystic fibrosis, mutations in the CFTR gene lead to the production of a defective protein that cannot effectively regulate the flow of salt and water. This leads to the accumulation of thick, sticky mucus in the lungs and other organs, which can lead to frequent infections and respiratory failure.
Trikafta is a combination of three drugs that target different aspects of CFTR function. Elexacaftor helps to stabilize the defective protein produced by the CFTR gene, while tezacaftor increases the amount of functional protein present on the cell surface. Ivacaftor helps to enhance the activity of the CFTR protein once it reaches the cell surface. By addressing these different aspects of CFTR function, Trikafta can improve lung function and reduce the occurrence of respiratory infections in patients with cystic fibrosis.
Dosing
The recommended dose and schedule for Trikafta will vary depending on the patient’s age and medical history. The drug is taken orally and may be given twice daily. Patients should follow their healthcare provider’s instructions for taking Trikafta and notify their provider if they miss a dose or experience any side effects.
Side Effects
Trikafta can cause a range of side effects, which may vary in severity depending on the patient’s individual treatment and medical history. Some common side effects of Trikafta include headache, diarrhea, and nausea. The drug can also cause more serious side effects, such as respiratory infections and liver abnormalities. Patients should report any symptoms they experience to their healthcare provider immediately, as prompt medical attention can help manage potential side effects.
Drug Interactions
Trikafta may interact with other medications, including some antibiotics and antifungal drugs. Patients should notify their healthcare provider of all medications they are taking, including prescription, over-the-counter, herbal, and vitamin supplements, to avoid potential drug interactions.
Contraindications
Trikafta is contraindicated in patients with a known hypersensitivity to any of its components. The drug may also not be recommended for use in patients with certain medical conditions, such as severe liver impairment.
Pregnancy and Breastfeeding
Trikafta is not recommended for use in pregnancy and may harm a developing fetus. Women of childbearing potential should use effective contraception during treatment and for at least one month after the last dose of Trikafta. It is unknown whether Trikafta is excreted in breast milk, and women should avoid breastfeeding while on this medication.
Conclusion
Trikafta (elexacaftor/tezacaftor/ivacaftor) is a combination medication used to treat cystic fibrosis in patients aged 12 years and older who have at least one F508del mutation in the CFTR gene. The drug works by targeting different aspects of CFTR function and can improve lung function and reduce respiratory infections in patients with cystic fibrosis. Trikafta is taken orally and may be given twice daily. Side effects may include headache, diarrhea, and nausea. The drug is contraindicated in patients with a known hypersensitivity to its components and may not be recommended for use in certain patients. Pregnant and breastfeeding women should avoid Trikafta unless the benefits outweigh the risks. Patients should discuss their specific treatment plan and potential risks and benefits of Trikafta with their healthcare provider.
Additional information
| Package | 84 tablets (fixed-dose combination containing 50 mg elexacaftor, 25 mg tezacaftor and 37.50 mg ivacaftor; 75 mg ivacaftor), 84 tablets (fixed-dose combination containing 100 mg elexacaftor, 50 mg tezacaftor and 75 mg ivacaftor; 150 mg ivacaftor) |
|---|
Be the first to review “Buy Trikafta (elexacaftor/tezacaftor/ivacaftor; ivacaftor) Online” Cancel reply
Related Products

Buy Breztri Aerosphere (budesonide/glycopyrrolate/formoterol fumarate) Online
Total Sales: 0
SKU: 215824

Buy Orkambi (lumacaftor/ivacaftor) Online
Total Sales: 0
SKU: 588142
Buy Symdeko (tezacaftor / ivacaftor) Online
Total Sales: 0
SKU: 262294
Buy Symkevi (tezacaftor/ivacaftor) Online
Total Sales: 0
SKU: 495385


Reviews
There are no reviews yet.