- Your cart is empty
- Continue Shopping

Product
Description:
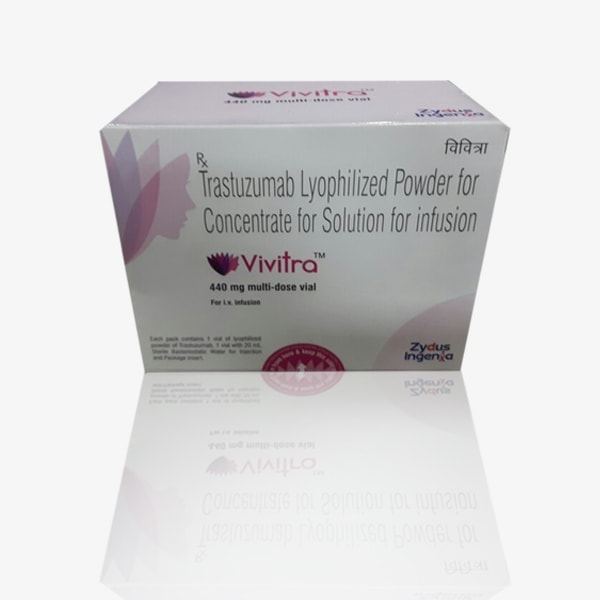
- Active Substance: Trastuzumab
- Vivitra contains the active ingredient trastuzumab, which is a monoclonal antibody used in the treatment of certain types of cancer, particularly HER2-positive breast cancer and gastric cancer.
- Strength: 440 mg Injection
- Vivitra is available in the form of an injection, and each vial typically contains 440 mg of trastuzumab. The strength represents the amount of the active substance in each dose.
- Medical Uses:
- Trastuzumab is primarily used in the treatment of HER2-positive breast cancer, a subtype of breast cancer that overexpresses the human epidermal growth factor receptor 2 (HER2) protein. It is also used in the treatment of HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma.
- Mode of Action:
- Trastuzumab works by targeting and binding to the HER2 protein on the surface of cancer cells, thereby blocking the signals that promote cancer cell growth and survival. It may also stimulate the immune system to attack HER2-positive cancer cells.
- Dosage and Administration:
- The dosage of Vivitra (trastuzumab) is determined by a healthcare professional based on the type and stage of cancer being treated, the patient’s overall health, and other factors. It is administered through intravenous (IV) infusion in a healthcare setting.
- Side Effects:
- Common side effects of trastuzumab may include nausea, diarrhea, fatigue, headache, and infusion reactions. It can also have more serious side effects such as cardiac toxicity and infusion-related reactions. Regular monitoring of heart function is essential during treatment.
- Precautions:
- Before using Vivitra, individuals should inform their healthcare provider about their medical history, existing medical conditions, and medications they are currently taking. Patients with pre-existing heart conditions may require close monitoring during treatment.
- Prescription Required:
- Yes, Vivitra is a prescription medication. It should only be used under the supervision of a qualified oncologist or healthcare professional.
- Duration of Treatment:
- The duration of treatment with trastuzumab is determined by the healthcare provider based on the response to treatment and the specific cancer being treated.
- Note:
- Trastuzumab is a targeted therapy drug that has significantly improved outcomes for patients with HER2-positive breast cancer and other HER2-positive cancers. However, its use requires careful monitoring due to potential side effects, particularly cardiac toxicity and infusion reactions.
It’s important to note that specific product information and medical guidance may vary, and the information provided here is a general description. Patients should refer to the product information leaflet provided with the medication and consult their healthcare provider for personalized advice.










Reviews
There are no reviews yet.