
Product
Buy Bacteriostatic Water for Injection Sterile Water and Non-Pyrogenic Online
$13.92
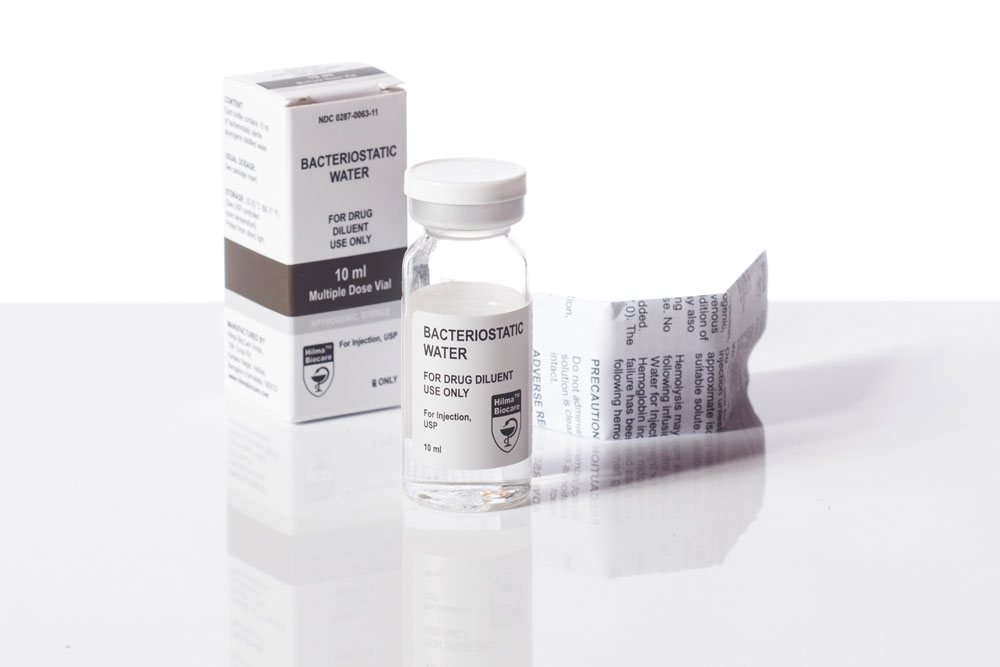
Bacteriostatic Water for Injection, USP, is a sterile water for injection that is non-pyrogenic and can be used multiple times. It includes a bacteriostatic preservative, benzyl alcohol, in a concentration of 0.9% (9mg/mL).
Add to cart
Buy Now
Bacteriostatic water is a specialized type of sterile water that has been rendered inhibitory to the growth and proliferation of bacteria. It is an essential component in various medical and pharmaceutical applications, primarily for the reconstitution and dilution of medications, particularly those administered by injection. The term “bacteriostatic” signifies its ability to prevent the multiplication of bacteria without actually killing them, making it suitable for preserving the integrity of certain pharmaceutical compounds.
Key features and characteristics of bacteriostatic water include:
1. Sterility: Bacteriostatic water is manufactured under stringent aseptic conditions to ensure it is free from any contaminants, including bacteria. This sterility is vital to prevent infections or complications when used for medical purposes.
2. Bacteriostatic Agent: Bacteriostatic water contains a specific antimicrobial agent, typically benzyl alcohol, which is added to inhibit bacterial growth. This agent hinders the replication of bacteria while preserving the solution’s sterility for a limited period.
3. Pharmaceutical Use: Bacteriostatic water is primarily used in the pharmaceutical industry to reconstitute or dilute medications in a safe and sterile manner. Medications that require reconstitution before use, such as powdered antibiotics or certain vaccines, benefit from the addition of bacteriostatic water to maintain their stability and safety.
4. Injection: It is commonly used for intramuscular and subcutaneous injections, particularly in clinical settings where multiple doses are required over an extended period.
5. Limitations: Bacteriostatic water should not be used for certain sensitive medications, as the bacteriostatic agent may interact with or affect their efficacy. For such drugs, a different type of diluent is recommended.
6. Shelf Life: Once opened, a vial of bacteriostatic water has a limited shelf life (usually around 28 days) before its sterility and the effectiveness of the bacteriostatic agent begin to decline.
7. Safety Precautions: Users should follow proper aseptic techniques when handling bacteriostatic water to maintain its sterility. Contaminated solutions can pose health risks, especially when administered by injection.
In summary, bacteriostatic water is a sterile solution containing a bacteriostatic agent that prevents the growth of bacteria while maintaining sterility. It is widely used in the pharmaceutical and medical fields for the safe reconstitution and dilution of medications, particularly for injections. Careful handling and adherence to safety protocols are crucial to ensure its effectiveness and safety in healthcare settings.







BevG –
I have been utilizing the Bacteriostatic Water for Injection, USP, regularly in my clinical and research practice, and it consistently delivers outstanding performance. This sterile, non-pyrogenic water includes 0.9% benzyl alcohol as a bacteriostatic preservative, which effectively inhibits bacterial growth, allowing multiple withdrawals from a single vial without compromising sterility. This feature significantly enhances convenience and reduces waste during procedures.
The solution is crystal clear and free from any contaminants or particulates, ensuring it meets stringent USP standards. It is highly compatible with various injectable peptides, hormones, and medications, providing a reliable diluent that maintains the integrity and potency of these compounds. The water mixes easily without causing precipitation or degradation, which is critical for both patient safety and treatment efficacy.
Additionally, the packaging is robust and secure, preventing any risk of contamination during shipping or storage. Overall, this Bacteriostatic Water for Injection is a dependable and essential component in any medical or laboratory setting where sterility, safety, and efficacy are paramount. I highly recommend it for practitioners and researchers who require a high-quality injectable diluent.
Sterline M. –
The water is crystal clear, easy to handle, and mixes well with peptides and other injectable substances. It maintains stability and sterility throughout its use, which is essential for patient safety and treatment efficacy. Packaging is professional and secure, ensuring product integrity upon arrival.
Overall, this bacteriostatic water meets the highest standards and is an excellent choice for any clinical or research setting requiring a dependable sterile diluent.